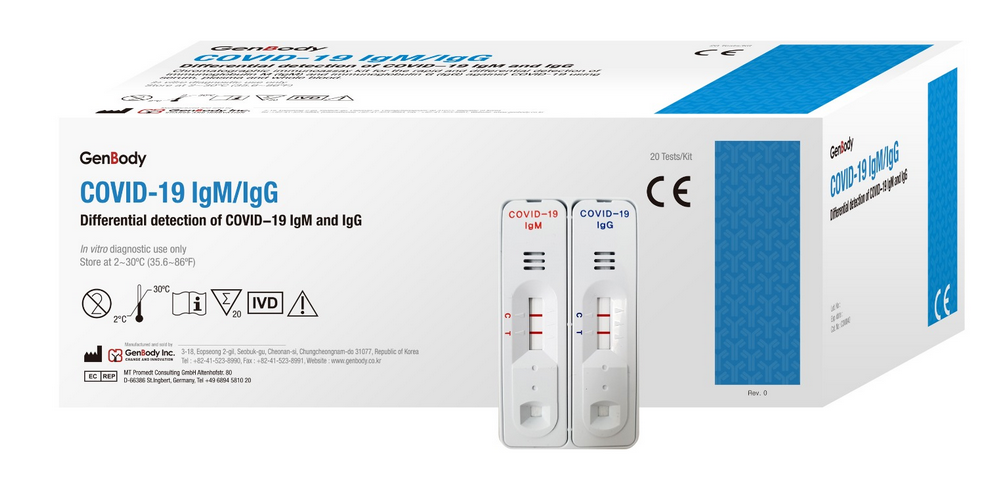
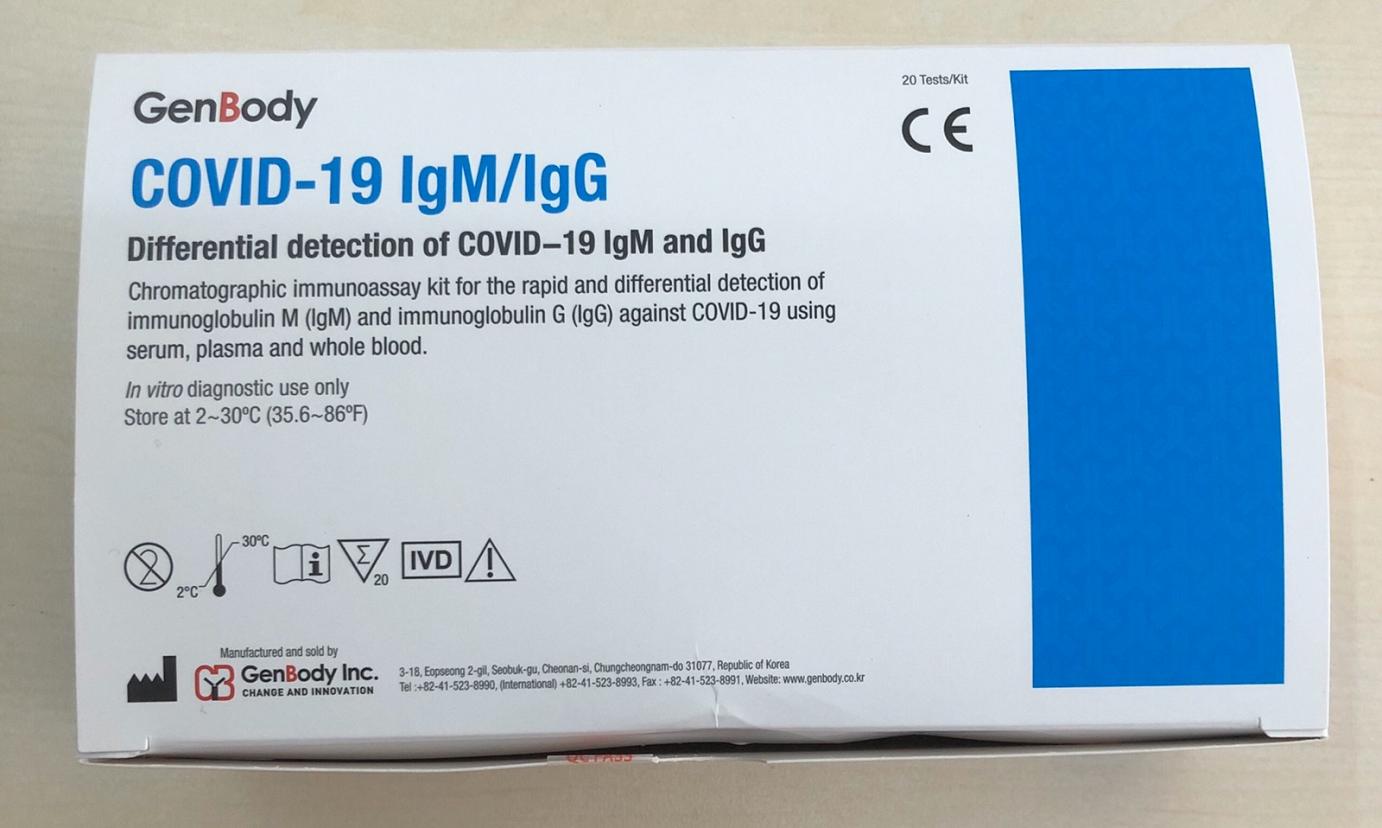
COVID-19 IgG/IgM Corenovirus Detection Test
The COVID-19 IgG/IgM (CE-IVD Approved) Test is a rapid test for qualitative and differential detection of IgG and IgM corovirus antibodies in human total blood (trichospecific), serum or plasma.
Product of GenBody Inc
GenBody is the actual manufacturer of the test (see manufacturer’s certificate on forms) and not OEM trafficker.
High specificity and sensitivity compared to PCR reference method
FEATURES GenBody COVID-19 IgG / IgM
-
- Solid-phase immunochromatography detection
- Fast, qualitative and differential detection of 2019 Novel Coronavirus IgG and IgM antibodies
- The test is done on tape, with a sample of total (trichospecific) blood, serum or plasma
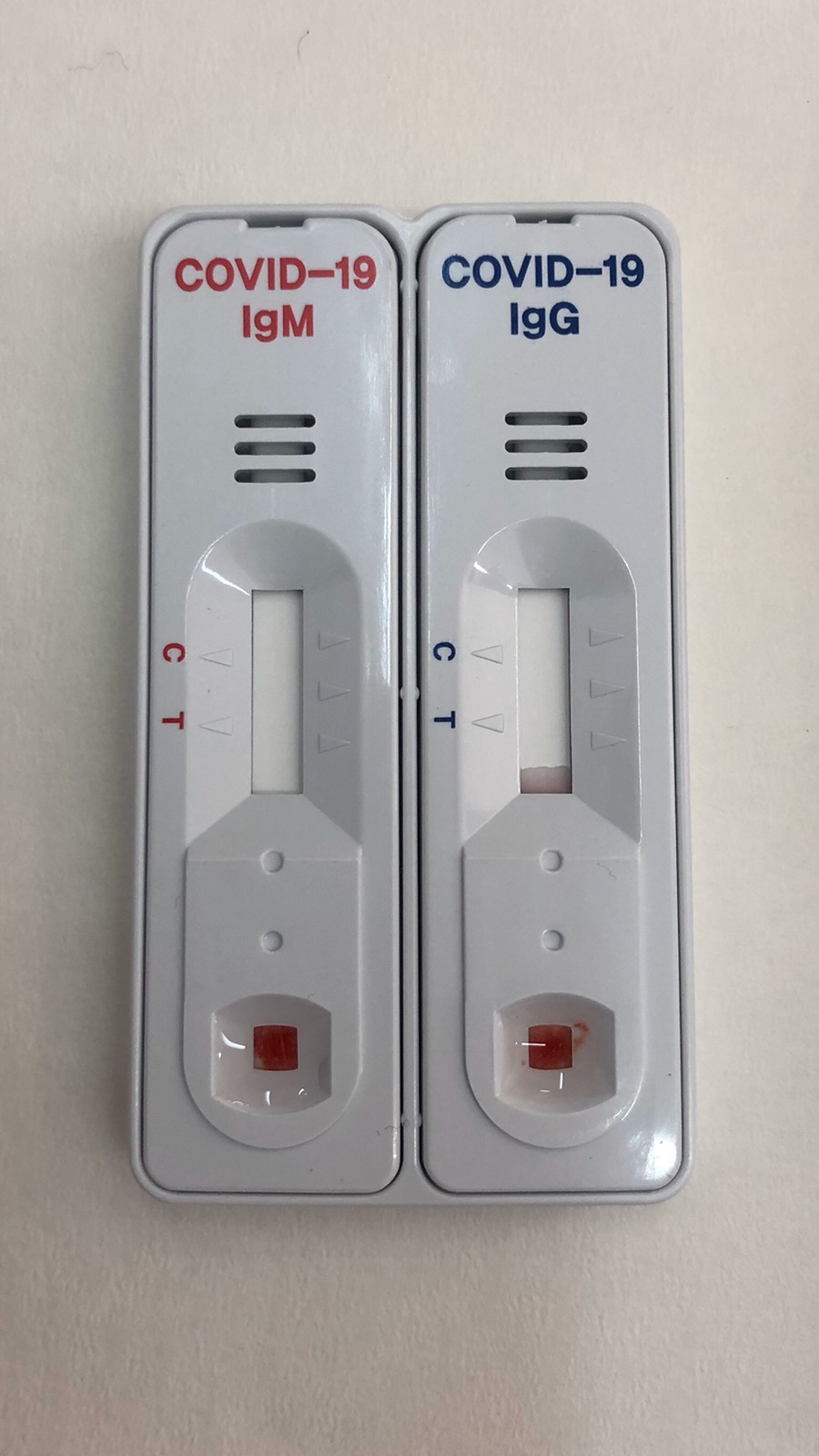
- As long as the sample is positive, virus antibodies (IgG/ IgM) detected on the cartridge in separate locations.
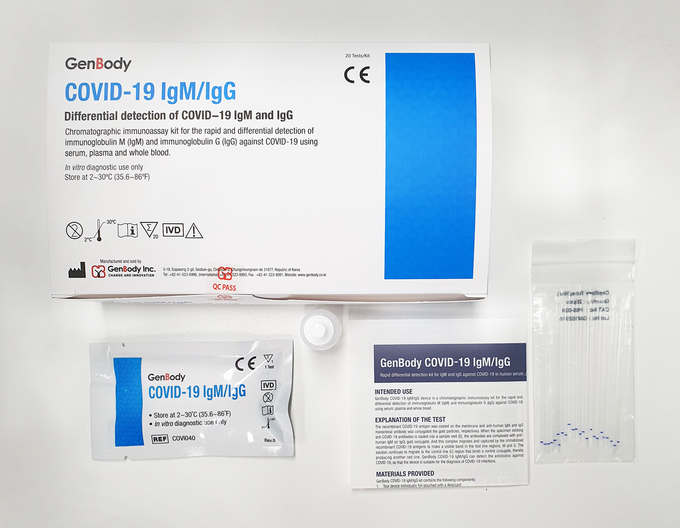
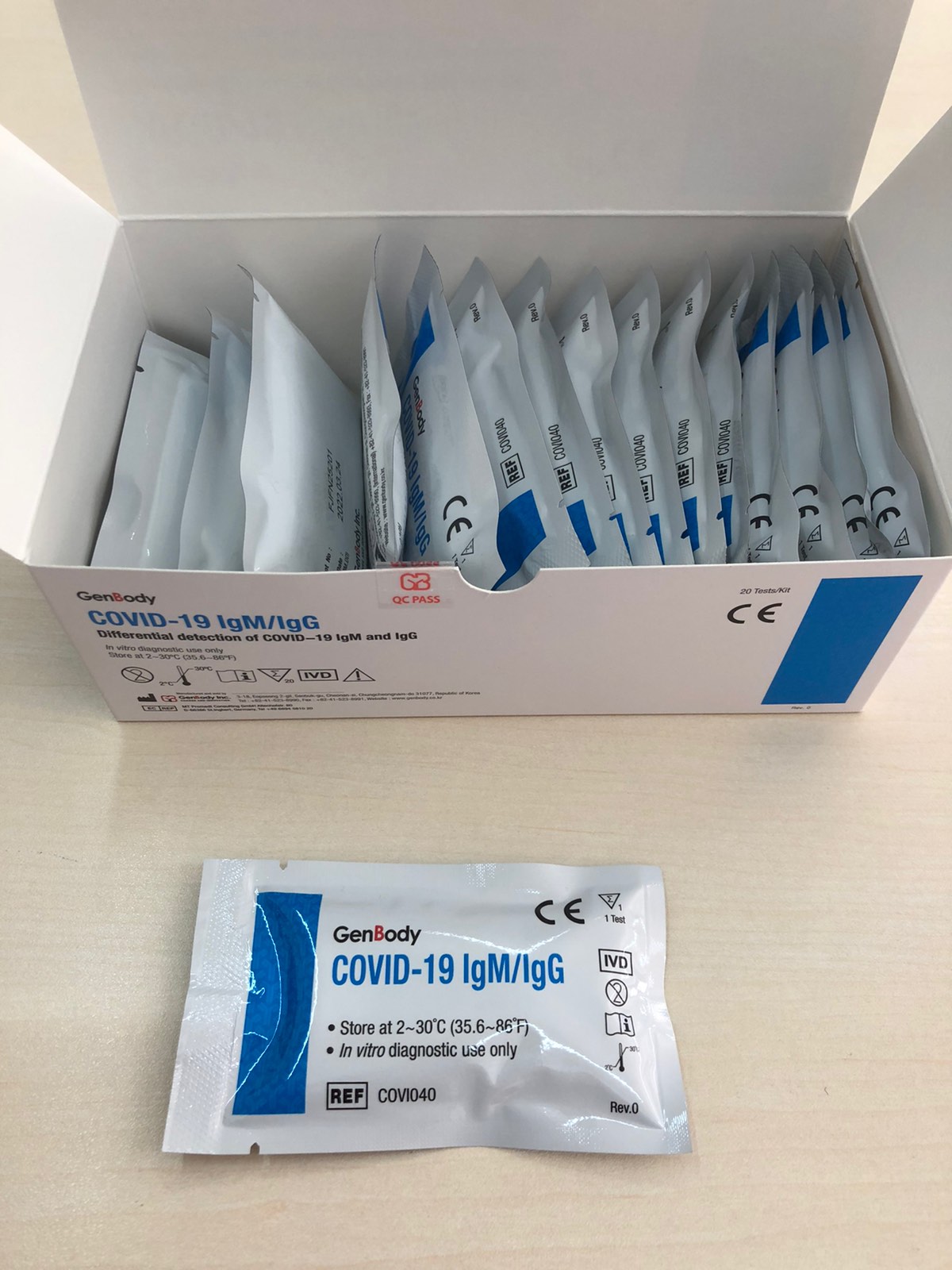
- The package includes 20 cartridges with eyedropper, desiccant & buffer.
- The control line is always converted from blue to red, thus confirming the sufficient sample volume and the correct functioning of the method.
- IgM antibodies against 2019 Novel Coronavirus can be detected 2-3 weeks after exposure to the virus.
- Ig antibodies against 2019 Novel Coronavirus will be detected for many years.
- In addition to “witness” control, which confirms the correct control procedure, the cartridge determines whether the patient has been diseased and has already developed antibodies (immunoglobulins G
).
- Getting it in just 10 minutes.
- Simple time-saving process
- Small samples, only 10μL of serum/plasma or 20μL of full blood samples
- High sensitivity and specialty
Klinitic data: COVID-19 IgG/IgM was evaluated in blood samples of patients who showed respiratory or pneumonia symptoms.
The results were compared with PCR and showed IgM sensitivity of 98.3% and 100% specificity, IgG sensitivity 99.1% and 100% specificity.
All relevant product forms (use & certificates of fitness) and relevant
study carried out in the Republic of Korea showing the significant assessment of antibodies in patients with a corona.
The fast diagnostic test GenBody COVID-19 IgM/IgG Quick Immunoassay significantly outperforms the others because in addition to its top sensitivity / speciality / reliability (sensitivity / specificity / overall accuracy) is the only quick test by which its user (thanks to the Reader Confiscope G20 available by GenBody for reading its diagnostics) can obtain not only a quality result (positive or negative) but also a quantitative result, i.e. antibody title, along with the relevant diagram that categorizes the result into [negative – Negative], [suspect to re-check – Gray Zone], [weakly positive – Weak Positive], [moderately positive – Moderate Positive] and [strongly positive – Strong Positive].
The existence of “Gray Zone” [suspect edited] is a major advantage as it detects even the suspicious samples in the early infection stages where the body has not yet begun to produce a detectable number of antibodies and which in the rest of the rapid tests would be given as “negative” .
Having (the user of the GenBody tests) in possession of the reader Confiscope G20 and not knowing the day of infection of the person tested for conovirus, he can perform repeated tests of his antibody level, in order to monitor them and finally receive data on both the course (evolution) of the disease and the behavior of the body of the affected person, both at the beginning of the disease and in its later course i.e. in the period when this person has an active infection (level of immunoglobulinS M) and in the future where the doctor can monitor the level of antibodies (degree of immunity) of the person who has been diagnosed (and has elevated levels in immunoglobulins-G), which will eventually allow us to monitor and finally learn better the disease.
COVID-19 IgG/IgM Coredetection Test covid-19 IgG/IgM Packaging of 20 Tests
Features
| Construction House: | GenBody |
| Recommended Use: | Professional Use |
| Warranty: | 1 Year |
| CE Certification: | EC notified body |
Video
Forms & Instructions
Vendor Information
- Store Name: Medical.GR
- Vendor: Medical.GR
-
Address:
Ιπποκράτους 142
Σπάτα
Attica
19004 - No ratings found yet!